 Graphical Abstract: Wiley
Graphical Abstract: WileyAbstract
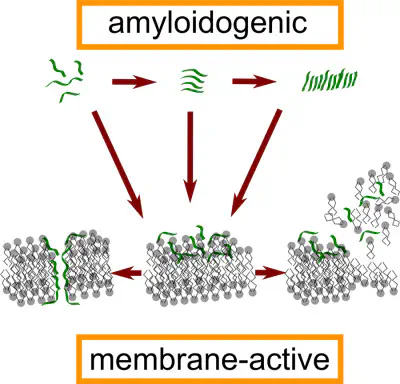
Amyloid fibrils are highly ordered, β-sheet rich forms of aggregated peptides and proteins that are associated with a variety of pathological human disorders, including Alzheimer’s and Parkinson’s diseases. Amyloid fibril-forming peptides may be functionally related to antimicrobial peptides, despite differing significantly in sequence and structure. Specifically, their interaction with lipid membranes has mechanistic similarities. The 17-amino acid peptide uperin 3.5 (U3.5) from an Australian amphibian is antimicrobial and amyloidogenic. Using a quartz crystal microbalance, we investigated the interaction of U3.5 with artificial membranes and found that (i) the membrane interaction of U3.5 is independent of the peptide’s aggregation state, (ii) the presence of cholesterol in the membrane dramatically alters peptide–membrane interaction leading to a transmembrane pore-like arrangement of U3.5, and (iii) electrostatic interaction is important for the membrane activity of U3.5 whereby removal of the positive charge at position 7 of U3.5 enhanced its fibrillar aggregation and ablated its membrane interaction, i.e. there is an inverse relationship between the antimicrobial and amyloidogenic properties of U3.5.