 Graphical Abstract: Wiley
Graphical Abstract: WileyAbstract
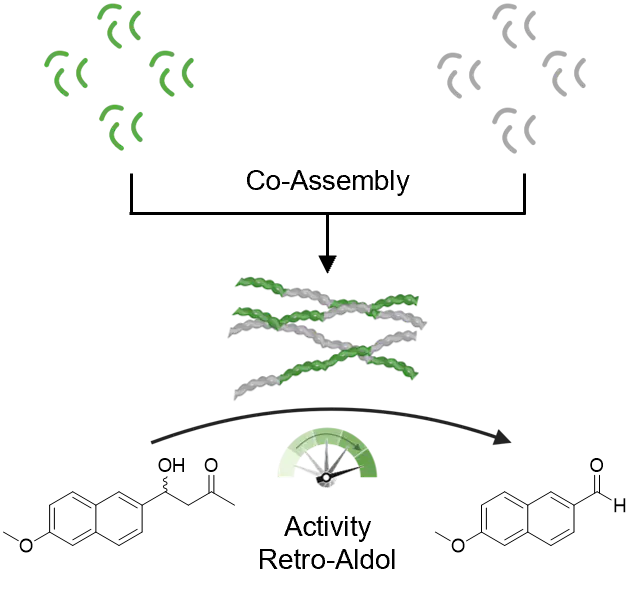
Short peptide sequences self-assemble into supramolecular structures through intermolecular interactions, creating a microenvironment in which chemical reactions can be catalyzed. In recent years, many peptide sequences have shown to demonstrate catalytic activity upon nanostructure formation, but the engineering of the catalytic microenvironment through co-assembly strategies have not been explored. We introduce a peptide sequence that gains retro-aldolase activity upon assembly to supramolecular peptide fibrils in aqueous buffer solution (pH 7.4). The catalytic activity is first optimized through synthetic sequence variation and the structure formation properties of the peptides are characterized. Co-assembly with inactive peptide sequences enables the up- or downregulation of the catalytic activity over a dynamic range, by modulating the likelihood for substrate interaction and thus the distance of the substrate to the nucleophilic lysine at the active site. It is observed that co-assemblies with positively charged sequences increase activity, whereas negatively charged peptide sequences decrease activity. We show that the emerging field of peptide-based catalysts can be further advanced by the engineering of the catalytic domain using heterogeneous supramolecular assembly.