Biomolecular Interactions at Interfaces
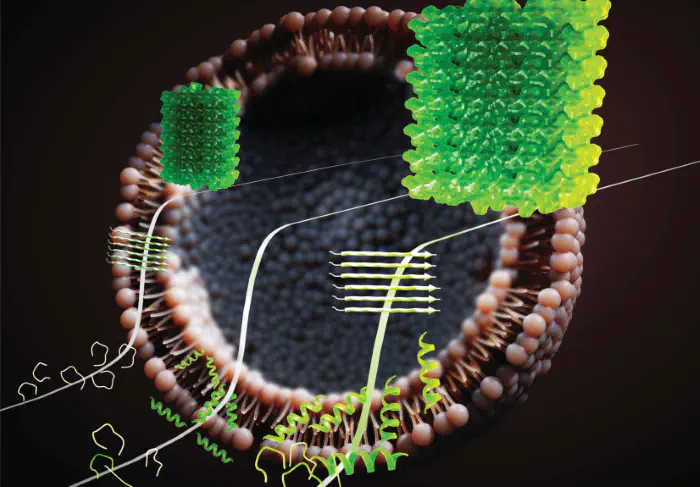
Interfaces play an active role in regulating biomolecular structure and function. Our research explores how peptides interact with biological and bio-inspired interfaces, including lipid membranes, nanoparticles, and soft matter surfaces. We examine how interfacial properties such as curvature, charge, and chemical functionality modulate peptide adsorption, self-assembly, and membrane activity. By elucidating interface-driven effects on peptide behavior, we aim to better understand processes such as antimicrobial activity, viral particle binding and concentration, and biomolecular transport in cellular environments.
Related Publications
Torsten John, Lisandra L Martin, Bernd Abel (2023). Peptide Self‐Assembly into Amyloid Fibrils at Hard and Soft Interfaces – From Corona Formation to Membrane Activity. Macromolecular Bioscience. https://doi.org/10.1002/mabi.202200576
Torsten John, Stefania Piantavigna, Tiara J A Dealey, Bernd Abel, Herre Jelger Risselada, Lisandra L Martin (2023). Lipid oxidation controls peptide self-assembly near membranes through a surface attraction mechanism. Chemical Science. https://doi.org/10.1039/D3SC00159H
Torsten John, Lisandra L Martin, Herre Jelger Risselada, Bernd Abel (2022). Curvature model for nanoparticle size effects on peptide fibril stability and molecular dynamics simulation data. Data in Brief. https://doi.org/10.1016/j.dib.2022.108598
Torsten John, Juliane Adler, Christian Elsner, Johannes Petzold, Martin Krueger, Lisandra L Martin, Daniel Huster, Herre Jelger Risselada, Bernd Abel (2022). Mechanistic insights into the size-dependent effects of nanoparticles on inhibiting and accelerating amyloid fibril formation. Journal of Colloid and Interface Science. https://doi.org/10.1016/j.jcis.2022.04.134
Torsten John, George W Greene, Nitin A Patil, Tiara J A Dealey, Mohammed A Hossain, Bernd Abel, Lisandra L Martin (2019). Adsorption of Amyloidogenic Peptides to Functionalized Surfaces Is Biased by Charge and Hydrophilicity. Langmuir. https://doi.org/10.1021/acs.langmuir.9b02063
Torsten John, Tiara J A Dealey, Nicholas P Gray, Nitin A Patil, Mohammed A Hossain, Bernd Abel, John A Carver, Yuning Hong, Lisandra L Martin (2019). The Kinetics of Amyloid Fibrillar Aggregation of Uperin 3.5 Is Directed by the Peptide’s Secondary Structure. Biochemistry. https://doi.org/10.1021/acs.biochem.9b00536
Torsten John, Anika Gladytz, Clemens Kubeil, Lisandra L Martin, Herre Jelger Risselada, Bernd Abel (2018). Impact of nanoparticles on amyloid peptide and protein aggregation: a review with a focus on gold nanoparticles. Nanoscale. https://doi.org/10.1039/C8NR04506B
Torsten John, Bernd Abel, Lisandra L Martin (2018). The Quartz Crystal Microbalance with Dissipation Monitoring (QCM-D) Technique Applied to the Study of Membrane-Active Peptides. Australian Journal of Chemistry. https://doi.org/10.1071/CH18129
Lisandra L Martin, Clemens Kubeil, Stefania Piantavigna, Tarun Tikkoo, Nicholas P Gray, Torsten John, Antonio N Calabrese, Yanqin Liu, Yuning Hong, Mohammed A Hossain, Nitin Patil, Bernd Abel, Ralf Hoffmann, John H Bowie, John A Carver (2018). Amyloid aggregation and membrane activity of the antimicrobial peptide uperin 3.5. Peptide Science. https://doi.org/10.1002/pep2.24052