Research
Research Vision
Our long-term goal is to establish predictive design principles that connect peptide sequence, environment, and interface chemistry to self-assembly pathways and biological function. By integrating biophysical experiments with molecular dynamics simulations, we aim to bridge fundamental mechanisms of peptide aggregation and membrane activity with disease-relevant insight and the rational design of functional biomolecular systems.
Methods and Expertise
- Molecular dynamics simulations (atomistic and coarse-grained)
- Biophysical characterization of peptide self-assembly
- Spectroscopic and surface-sensitive techniques (e.g., QCM-D, fluorescence)
- Interface, nanoparticle, and membrane models
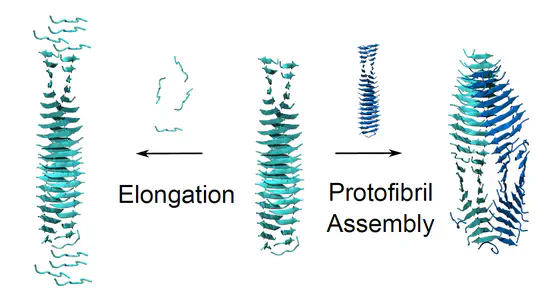
Mechanisms of Peptide Self-Assembly
We investigate the molecular mechanisms governing peptide self-assembly, with a particular focus on aggregation pathways, polymorphism, and β-sheet-rich amyloid fibrils. Using spectroscopy, surface-sensitive techniques, and molecular dynamics simulations, we study how peptide sequence, solvent conditions, and confinement influence nucleation, fibril growth, and higher-order organization. These studies are directly relevant to understanding aggregation processes associated with age-related and neurodegenerative diseases, while also providing a mechanistic foundation for the rational design of peptide-based materials.
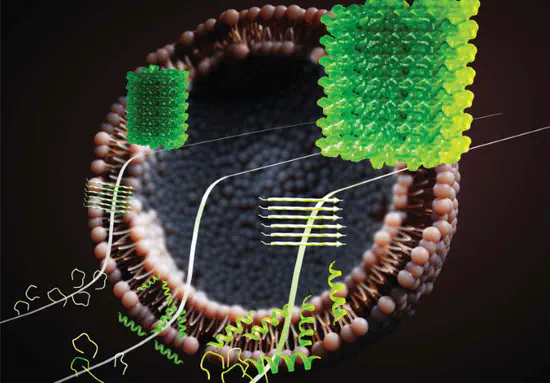
Biomolecular Interactions at Interfaces
Interfaces play an active role in regulating biomolecular structure and function. Our research explores how peptides interact with biological and bio-inspired interfaces, including lipid membranes, nanoparticles, and soft matter surfaces. We examine how interfacial properties such as curvature, charge, and chemical functionality modulate peptide adsorption, self-assembly, and membrane activity. By elucidating interface-driven effects on peptide behavior, we aim to better understand processes such as antimicrobial activity, viral particle binding and concentration, and biomolecular transport in cellular environments.
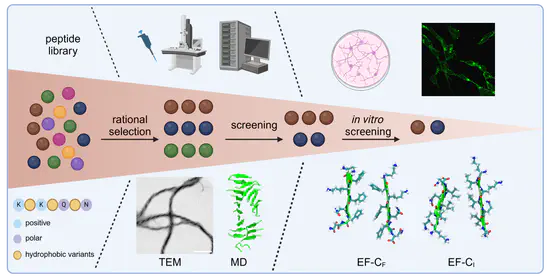
Design of Functional Bionanomaterials
Building on our mechanistic understanding of peptide self-assembly and interfacial interactions, we engineer functional bionanomaterials. Our work includes peptide fibrils as well as hybrid systems combining peptides with nucleic acids, such as DNA origami, to create materials with defined structure and emergent function. These systems are explored for applications in areas including biosensing, targeted delivery, and modulation of cell-material interactions, while remaining closely connected to fundamental questions in biophysical chemistry.