Mechanisms of Peptide Self-Assembly
We investigate the molecular mechanisms governing peptide self-assembly, with a particular focus on aggregation pathways, polymorphism, and β-sheet-rich amyloid fibrils. Using spectroscopy, surface-sensitive techniques, and molecular dynamics simulations, we study how peptide sequence, solvent conditions, and confinement influence nucleation, fibril growth, and higher-order organization. These studies are directly relevant to understanding aggregation processes associated with age-related and neurodegenerative diseases, while also providing a mechanistic foundation for the rational design of peptide-based materials.
Related Publications
Yu-Liang Tsai, Primiana Cavallo, Qi Lu, Jiyao Yu, Christopher P Ender, Julian Link, Katrin Amann-Winkel, Kristina Endres, Christopher V Synatschke, Torsten John (2025). Design of the Hydrophobic Core of Self-Assembling Peptide Fibrils for Enhanced Neural Regeneration. Small Science. https://doi.org/10.1002/smsc.202500224
Svenja Weigold, Kerstin Brödner, Torsten John, Jan Freudenberg, Uwe H F Bunz, Tanja Weil, George Fytas, Klaus Müllen (2025). Self‐Assembly of Amphiphilic Polyphenylene Dendrimers with Different Surface Functionalization in Solvent/Non‐Solvent Mixtures. Macromolecular Chemistry and Physics. https://doi.org/10.1002/macp.202400431
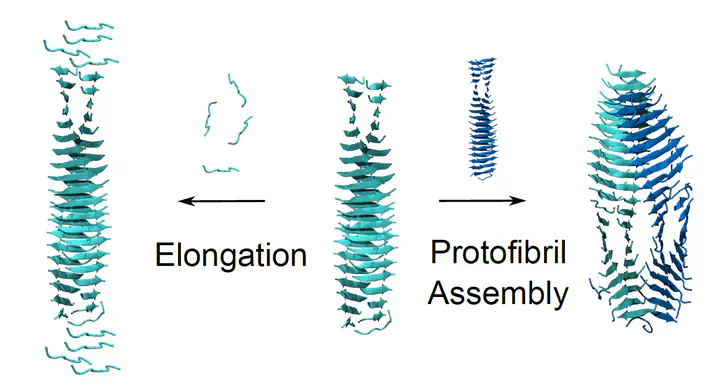
Torsten John, Aldo Rampioni, David Poger, Alan E Mark (2024). Molecular Insights into the Dynamics of Amyloid Fibril Growth: Elongation and Lateral Assembly of GNNQQNY Protofibrils. ACS Chemical Neuroscience. https://doi.org/10.1021/acschemneuro.3c00754
Torsten John, Juhaina Bandak, Nilushiya Sarveson, Claudia Hackl, Herre Jelger Risselada, Andrea Prager, Christian Elsner, Bernd Abel (2020). Growth, Polymorphism, and Spatially Controlled Surface Immobilization of Biotinylated Variants of IAPP 21–27 Fibrils. Biomacromolecules. https://doi.org/10.1021/acs.biomac.9b01466
Torsten John, Tiara J A Dealey, Nicholas P Gray, Nitin A Patil, Mohammed A Hossain, Bernd Abel, John A Carver, Yuning Hong, Lisandra L Martin (2019). The Kinetics of Amyloid Fibrillar Aggregation of Uperin 3.5 Is Directed by the Peptide’s Secondary Structure. Biochemistry. https://doi.org/10.1021/acs.biochem.9b00536
Lisandra L Martin, Clemens Kubeil, Stefania Piantavigna, Tarun Tikkoo, Nicholas P Gray, Torsten John, Antonio N Calabrese, Yanqin Liu, Yuning Hong, Mohammed A Hossain, Nitin Patil, Bernd Abel, Ralf Hoffmann, John H Bowie, John A Carver (2018). Amyloid aggregation and membrane activity of the antimicrobial peptide uperin 3.5. Peptide Science. https://doi.org/10.1002/pep2.24052