Design of the Hydrophobic Core of Self-Assembling Peptide Fibrils for Enhanced Neural Regeneration
 Graphical Abstract: Wiley
Graphical Abstract: WileyAbstract
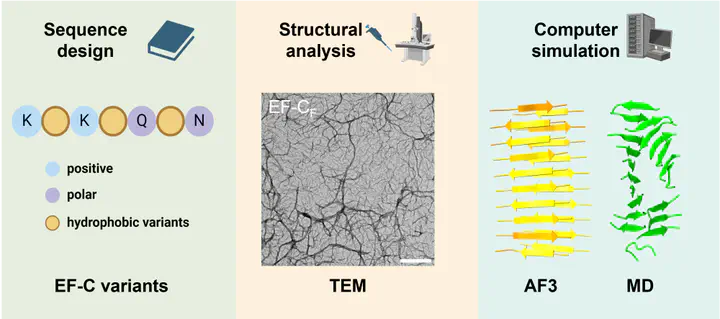
Neurons have limited self-repair ability, and typical treatment approaches for damaged tissue rely on surgery. Hindered by the lack of donor tissues and the complex neural environment, there is great interest in developing biomaterials to support neural regeneration. Self-assembling peptides with fibrous structures mimicking the extracellular matrix have shown great potential as neurosupportive biomaterials. Previously, we identified peptide sequences derived from enhancing factor-C (EF-C) that are neurotrophic without additional supplements. Here, a library of nine EF-C variants is designed by varying the hydrophobic core of the peptide backbone to elucidate its influence on self-assembly and bioactivity. The physicochemical properties of these variants, including secondary structure and morphology, are thoroughly analyzed. Furthermore, molecular dynamics simulations based on AlphaFold 3 models are conducted, providing theoretical insights that explain the differential assembly and stability of EF-C variants. Subsequently, the peptides are tested for bioactivity in a neuroblastoma cell line (SH-SY5Y) to establish structure–property relationships. The structure-forming EF-C variants, particularly those featuring phenylalanine and isoleucine, are neurotrophic toward SH-SY5Y cells, shown by enhanced ATP levels. The combination of experimental and computational methods provides a strategy for the accelerated design of neuro-regenerative peptides.